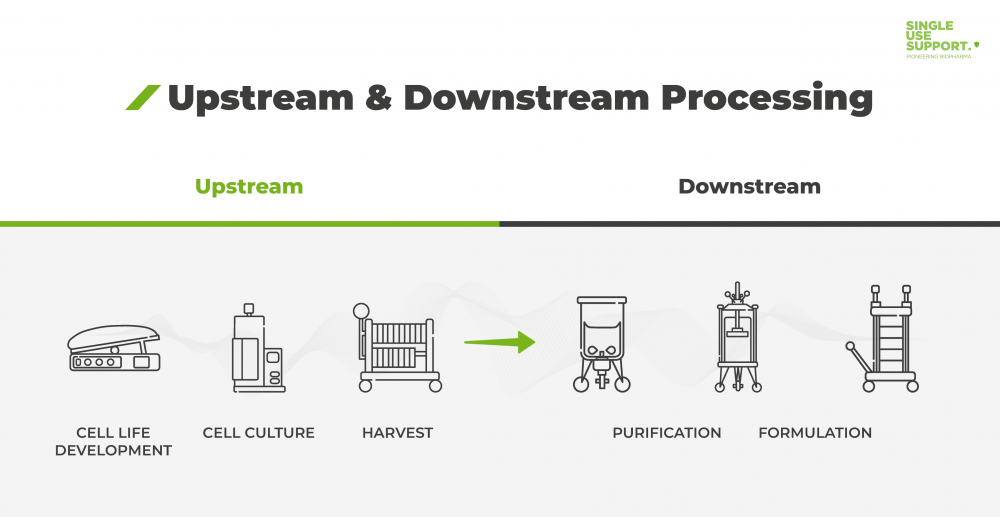
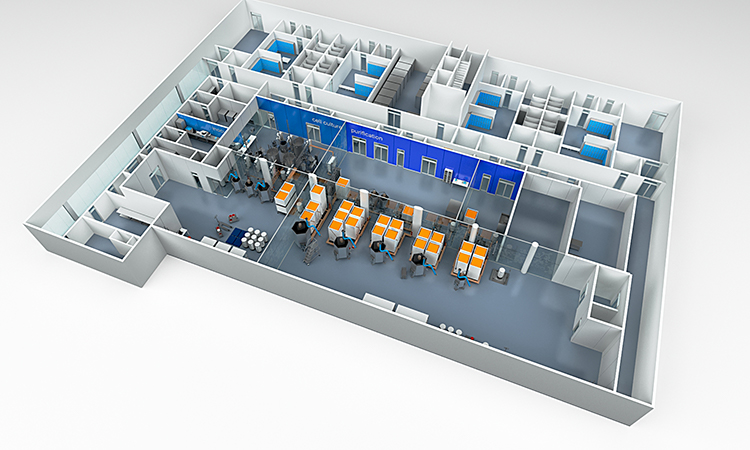
GXP BIOPHARMA, LLC is a privately held Biopharma Contract Development Manufacturing Organization (CDMO) company for producing new Biopharma Products for Clinical Trials by its clients. GXP BIOPHARMA will service universities, Incubator Companies, and small and large Biotechnology industry developing Biopharma products for health care. GXP-BIOPHARMA production includes Research, Diagnostic, Therapeutic and Combination Drug-Device products.
GXP-BIOPHARMA can provide Regulatory Submissions (IND, Investigational New Drug Submissions) for Clinical Trials.
GXP-BIOPHARMA can provide start up Biopharma companies with GMP Incubator Laboratory space.
Commercial Manufacturing will be performed by GXP BIOPHARMA corporate collaborators.